Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 24/11/2020
The current COVID-19 pandemic caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) counts more than 58 million recorded cases (www.WHO.org; November 23rd 2020), highlighting the urgent demand for the development and identification of a specific antiviral treatment.
Amongst potential candidates, the main protease (Mpro, 3CLpro, nsp5) has become a promising drug target as it represents a key protein in the lifecycle of SARS-CoV-2 with a pivotal role in mediating viral replication and transcription. Importantly, it significantly differs from human proteases. Consequently, its inhibition allows to prevent the formation of functional viral particles without affecting the human host proteases.
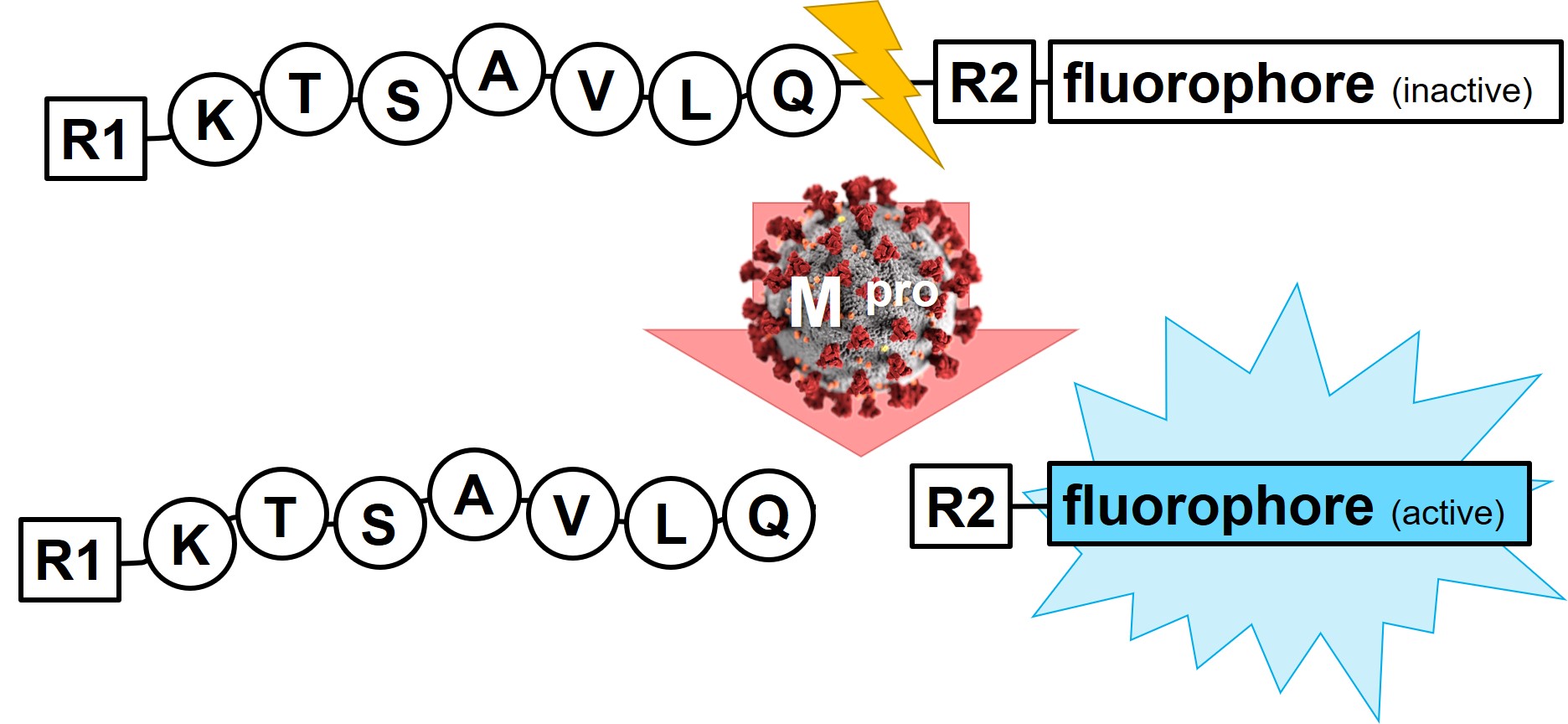
Potential inhibitors of SARS-CoV-2 Mpro can easily be screened for their activity by using a fluorescence read-out assay. Iris Biotech’s portfolio comprises two compounds, which can be used for activity determination and inhibitor screening of the main protease.

SARS-CoV-2 Mpro substrates provided by Iris Biotech. Cleavage site indicated by an orange arrow.
Prior to enzymatic cleavage, the Edans fluorescence of LS-4180 is quenched by the closely located Dabcyl based on a FRET-process. Upon cleavage and excitation at 340 nm, fluorescence emission can be read-out at 490 nm.
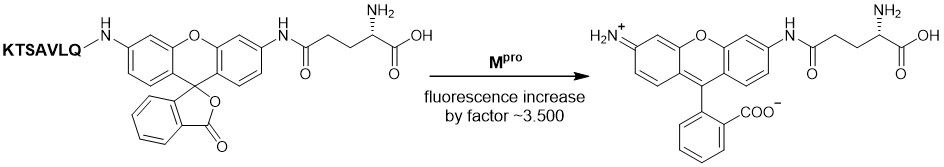
The rhodamine 110-based substrate LS-4190 benefits from red-shifted excitation (492 nm) and emission (529 nm) wavenlenghts, as well as from an improved signal-to-noise ratio. In addition, it produces less interference with potential inhibitors in screening assays. This leads to a higher sensitivity compared to the Dabcyl/Edans substrate. In consequence, lower substrate amounts are required for successful assays.
Interested in purifying peptide libraries? Please see our Belyntic high-throughput peptide purification kits BYR4820 and BYR9610. These kits enable the massive parallelization of peptide manufacturing and allow to create purified peptide libraries using the catch-and-release technology in 48- or 96-well filter plates.
References: