Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 04/04/2023

In general, the conversion rate of a coupling reaction in solid phase peptide synthesis or solution phase peptide synthesis is defined by several parameters, such as:
In this blog, we are focusing on the choice of the coupling reagent. A primary amine and a carboxylic acid group do not spontaneously undergo an amide bond formation with each other. Efficient combination of e.g. two amino acid residues to form a peptide bond requires certain activation of the carboxyl group. Procedures allowing this combination are referred to as coupling methods. Coupling involves the attack of the amino group of one residue at the carboxy-group of the other residue, which has been activated by the introduction of an electron withdrawing group. However, this activation step, along with the next coupling reaction, leads to potential loss of chiral integrity at the carboxyl residue undergoing activation.
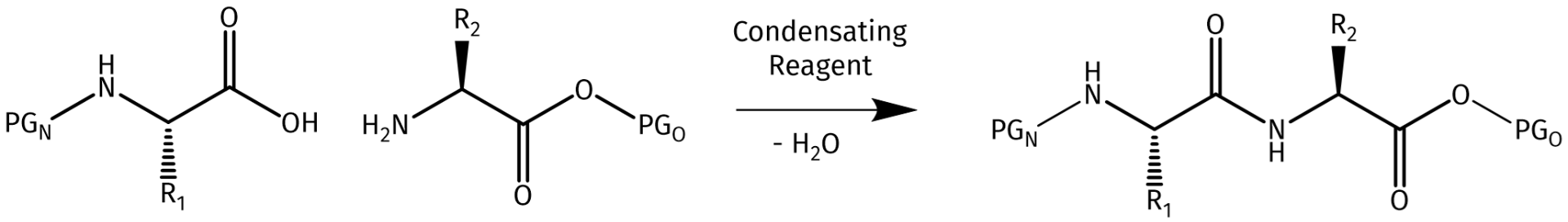
Schematic illustration of the amide bond formation (condensation) between two amino acid residues.
Defining standard coupling conditions is a common procedure when developing a new synthetic route. In case of incomplete coupling, either coupling conditions can be optimized, or double or even triple coupling with the same conditions can be applied.
In the following section we describe reaction mechanisms of different classes of coupling reagents with an emphasis on potential side reactions leading to racemization or other possible impurities.
Carbodiimides & Activator
The concept of coupling reagents was introduced by Sheehan and Hess using N,N’-dicyclohexylcarbodiimide (DCC), representing a breakthrough in peptide synthesis. In combination with additives, racemization is being suppressed, while yield increases. In an initial step, an O-acyl intermediate is formed which can act as active ester and may lead to the desired amide bond.
Besides, addition of activators suppresses side reactions such as O→N acyl shift, or dehydration of asparagine and glutamine residues can be suppressed.

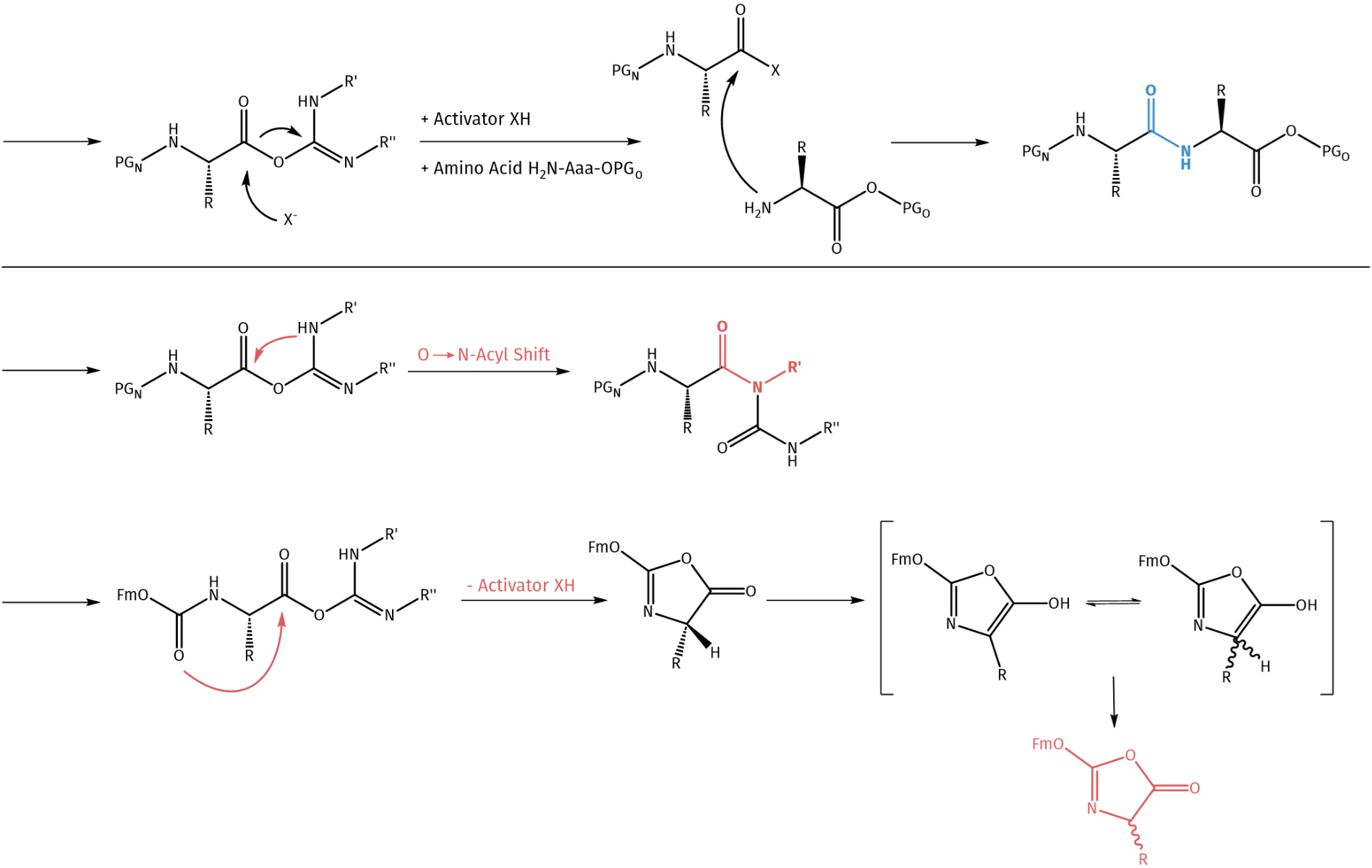
DCC-mediated amide bond formation and possible byproduct formation without activator addition.
However, common activators like HOBt or HOAt have been banned from the market due to their explosive potential and are replaced by alternatives, like pentafluorophenol (Pbf), N-hydroxysuccinimide, (HOSu/NHS), ethyl cyano(hydroxyimino)acetate (OxymaPure®, RL-1180) or 2-mercaptobenzothiazole (2-MBT, RL-1165).
Uronium and Phosphonium Salts
The need for stronger activating reagents – compared to carbodiimides – led to the introduction of stand-alone aminium, uranium, and phosphonium salts, which have become the preferred coupling choice in solution- and solid-phase peptide synthesis.
→ You are looking for standard coupling protocols? Download our Resin Guideline!
Formation of Acid Halides
In 1903, acid chlorides were first introduced for peptide coupling by Emil Fischer. Nowadays, this route is hardly used due certain drawbacks, e.g. hydrolysis, racemization, cleavage of protecting groups, and other side reactions, e.g. N-carboxy anhydride formation. More practical is the use of acid fluorides, both in solution and solid phase chemistry, as they are more stable to hydrolysis and more reactive towards amines compared to acid chlorides. Acid fluorides show similar reactivity as activated esters and are especially suited for the coupling of sterically hindered R,R-disubstituted amino acids. The most common reagent used to generate acid fluorides is TFFA (RL-1077).
→ See our related products at the bottom of this page!
→ You are interested in all our coupling reagents? Browse our website!
References:
Synthese von Peptiden. Methoden der organischen Chemie; E. Wünsch, E. Müller; Houben-Weyl Bd. XV/1, Stuttgart, Georg Thieme Verlag: 1974; 39.
Peptides: Chemistry and Biology; N. Sewald, H.-D. Jakubke; Wiley-VCH Verlag, Weinheim, 2009. ISBN: 978-3-527-31867-4
Re-evaluating the stability of COMU in different solvents; A. Kumar, Y. E. Jad, B. G. de la Torre, A. El-Faham, F. Albericio; Journal of peptide science : an official publication of the European Peptide Society 2017; 23: 763-768. https://doi.org/10.1002/psc.3024
Peptide coupling reagents, more than a letter soup; A. El-Faham, F. Albericio; Chem Rev 2011; 111: 6557-602. https://doi.org/10.1021/cr100048w
Oxyma: an efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion; R. Subiros-Funosas, R. Prohens, R. Barbas, A. El-Faham, F. Albericio; Chemistry 2009; 15: 9394-403. https://doi.org/10.1002/chem.200900614
The uronium/guanidinium Peptide coupling reagents: finally the true uronium salts; L. A. Carpino, H. Imazumi, A. El-Faham, F. J. Ferrer, C. Zhang, Y. Lee, B. M. Foxman, P. Henklein, C. Hanay, C. Mugge, H. Wenschuh, J. Klose, M. Beyermann, M. Bienert; Angew. Chem. Int. Ed. Engl. 2002; 41: 441-5. https://doi.org/10.1002/1521-3773(20020201)41:3<441::aid-anie441>3.0.co;2-n
Choosing the Right Coupling Reagent for Peptides: A Twenty-Five-Year Journey; F. Albericio, A. El-Faham; Org. Process Res. Dev. 2018; 22(7): 760-772. https://doi.org/10.1021/acs.oprd.8b00159
A New Method of Forming Peptide Bonds; J. C. Sheehan, G. P. Hess; J. Am. Chem. Soc. 1955, 77(4): 1067-1068. https://doi.org/10.1021/ja01609a099