Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
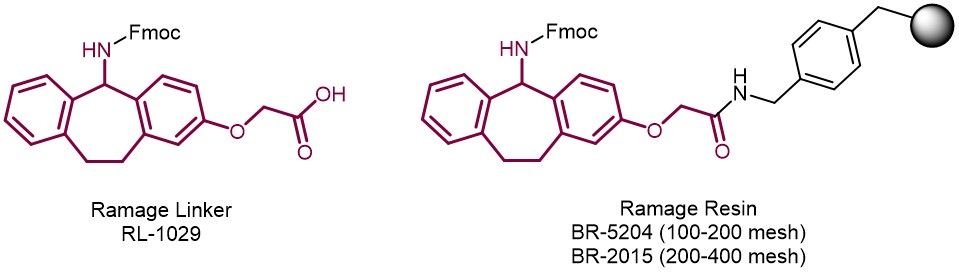
Continue to Iris Biotech GmbHSend request to US distributorChemical name: (R,S)-2-{[5-(9-Fluorenylmethyloxycarbonylamino)-dibenzo[a,d]cycloheptane-2-yl]oxy}-acetic acid // Synonyms: Fmoc-Suberol
Starting at $181.25
Alternative to the commonly used Rink-amide linker. The three-circular structure of the Ramage linker prevents fragmentation of the linker during cleavage from the resin followed by back alkylation. A common phenomenon observed during peptide synthesis with the open structure of the Rink-amide linker. Hence, Ramage linker delivers peptides with higher purity and less impurities, than peptides produced with the Rink-amide linker. Ramage resin is particularly recommended for C-terminal Phe, Tyr and Ile. After attachment to a resin and Fmoc removal standard SPPS can be carried out with conventional Fmoc/tBu protocols Peptide amides can be released from the resin with 50% TFA in DCM or 95% aqueous TFA.
Potassium channel modulation by a toxin domain in matrix metalloprotease 23; S. Rangaraju, K. K. Khoo, Z. P. Feng, G. Crossley, D. Nugent, I. Khaytin, V. Chi, C. Pham, P. Calabresi, M. W. Pennington, R. S. Norton and K. G. Chandy; J Biol Chem 2010; 285: 9124-36. https://doi.org/10.1074/jbc.M109.071266
An immunomodulator used to protect young in the pouch of the Tammar wallaby, Macropus eugenii; R. V. Baudinette, P. Boontheung, I. F. Musgrave, P. A. Wabnitz, V. M. Maselli, J. Skinner, P. F. Alewood, C. S. Brinkworth and J. H. Bowie; FEBS J 2005; 272: 433-43. https://doi.org/10.1111/j.1742-4658.2004.04483.x
Evaluation of some fluorogenic substrates for continuous assay of aminopeptidase P; S. J. Hawthorne, P. Harriott, J. Lim, A. J. Turner, B. Walker and C. H. Williams; Anal Biochem 1997; 253: 13-7. https://doi.org/10.1006/abio.1997.2320
Design of a versatile linker for solid phase peptide synthesis: Synthesis of C-terminal primary/seconary amides and hydrazides; R. Ramage, S. L. Irving and C. McInnes; Tetrahedron Letters 1993; 34: 6599-6602. https://doi.org/10.1016/0040-4039(93)88115-y
Do you need larger quantities for your development or production?
Please send me more information about